Announcing #RealTimeChem Week 2022 – #Inclusivechem (7th November-13th November 2022)
Hi Real Time Chemists,
We’re back! It’s #RealTimeChem‘s 10th Anniversary and we’ve teamed up with C&EN magazine once again to bring you another Week of chemistry-related fun.

For those not already in the know, #RealTimeChem Week is a 7 day themed event to help raise awareness of the #RealTimeChem chemistry community on Twitter and encourage as many chemists as possible to connect with each other by tweeting about their chemistry as they are doing it. During the week there are events to take part in with prizes on offer, so it’s the perfect week to jump in and share some chemistry with your fellow chemists.
If you are completely new to the community and want to know more about #RealTimeChem in general, then follow this link to the regular “About…” page, where you can learn all about the project and the community.
I’m sure you have questions, you are scientists. Here’s some common questions and the related answers below, including details on this year’s theme, events and prizes:
When is it?
It runs all day, for all 7 days.
How do I take part again?
Just tweet about chemistry using the hashtag #RealTimeChem. Simple as that. You should also use this year’s theme hashtag #Inclusivechem. That hashtag will also be monitored by me (& our sponsors at C&EN) during the event. We also have a series of other hashtag based daily roll calls during the week to highlight different communities that you can be involved in, so check those out below.
What is this year’s theme?
#RealTimeChem has a global community with Tweeters from all over the world. They come from a diverse range of cultures and with a huge variety of backgrounds and experiences.
This year we have chosen to both recognise and celebrate this diversity using the hashtag #Inclusivechem. It is so important that we value every member of this community and show that all are welcome. It is our greatest strength. For chemistry to prosper and help the world, it must be inclusive and accessible to all.
Chemistry is for everybody. Let’s celebrate what makes it great. All of you.
Throughout the week we and our friends at C&EN magazine will be asking:
- What does #InclusiveChem mean for you?
- Show us your #RealTimeChem! Where do you find chemistry around you?
- Give a shout out to a mentor, teacher, or colleague who has opened doors in your #InclusiveChem journey
- What are your #FlagsOfChem?
- What is your favorite queer molecule and why?
- What languages do you use to communicate your science?
- How have you changed in the last 10 years of #RealTimeChem? – how did it start and how is it going?
What are the Daily Events?
In addition to above we will be celebrating #InclusiveChem throughout the week with the following daily roll calls to highlight diversity in our chemistry community:
Nov. 7: #InclusiveChem kick off and Fly your #FlagsOfChem – this can be any flag that you feel is important to who you are – you don’t need to just chose one there are lots! You’ll find quite a few on our banner this year.
Nov. 8: #BlackInChem in partnership with @BlackInChem & @NOBCChE
Nov. 9: #DisabledInChem in partnership with @DisabledInSTEM
Nov. 10: #LatinXinChem in partnership with @LatinXchem
Nov. 11: #QueerInChem in partnership with @PrideinSTEM
To take part in a roll call all you need to do is introduce yourself and/or your chemistry using the relevant hashtag. You can just tweet hello, include photos or video. It’s up to you. We’d love for you to take part and help showcase the wonderful diversity in our community. We’ll also be making shout outs all week to chemists in these communities via @RealTimeChem – we’d love to hear yours too. Do also check out our partners and the great I&D work that they are doing within chemistry.
What are the prizes during #RealTimeChem Week 2022?
Like in previous years there will be prizes for #RealTimeChem Week tweets. However, this year to celebrate 10 years of #RealTimeChem and Inclusion and Diversity in the chemical science, we will be giving away 100 limited edition mugs (yes folks the mugs are baaaaack) featuring the #Inclusivechem art by Andy Brunning.
Winners this year will be selected at random from those who tweet during the week using #InclusiveChem, so everyone has an equal chance of winning!
C&EN magazine will also be running a story online highlighting some of their favourite Tweets during the event, so make it a good one.
Winners will be asked to provide name and address details via DM or email after the event.
#RealTimeChem Week Adverts
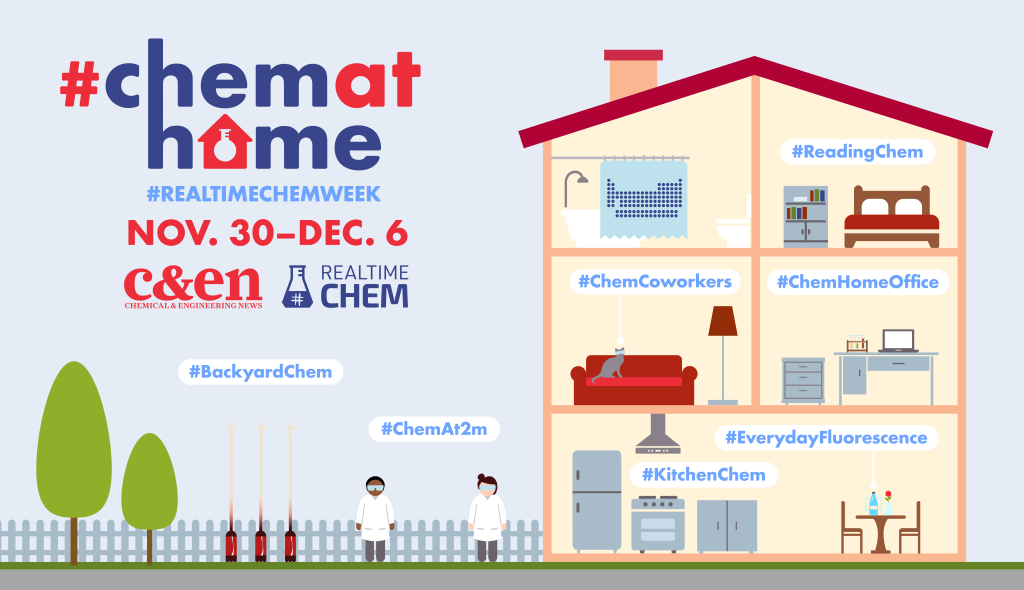
Below you will find banners to help you share the word about #RealTimeChem Week 2022. Our graphics whizz Andy Brunning (www.compoundchem.com) is back this year with some fantastic new banners to promote and celebrate #inclusivechem.
Feel free to share these adverts far and wide.






Want to run a competition or event?
There is always room for more chemistry-based fun. If you would like to run an event or competition during #RealTimeChem Week, then please get in touch with me via realtimechem@gmail.com and I’ll be happy to chat about the possibilities.
See you all during the week!
-Doctor Galactic-