Disclaimer: Obviously, I don’t own BatmanTM or any of the other stuff discussed in this blog post. This is just for fun, so Warner Brothers and DC, please don’t sue. The same goes for all the owners of the trademarked compounds mentioned.
So the final instalment of Christopher Nolan’s Batman trilogy, The Dark Knight Rises, has been released bringing to an end the best trilogy since The Lord of the Rings. I promised the other week (yeah I know it was aaaaaaages ago *grovel*) that I’d write something more geek related and so here it is a little piece on some of the chemistry supporting Bruce Wayne’s one man war on the criminals of Gotham City.
Batman has always been renowned for his use of gadgets and state of the art technology, which is probably for the best because he’s a superhero without…well, the super bit. Nolan’s films have taken a grittier, more realistic take on the Dark Knight of comic book lore and that’s the version I’m going to be looking at here.

See? Look how serious Christian Bale is taking this. That’s his serious face.
This started out as a short blog post and it got away from me somewhere in the middle, so I’ll be breaking it into two parts. This first post will concern Batman’s most obvious bit of kit: The Batsuit itself. The second will focus on his other gadgets (in that nifty belt shown below).

Like all scientists worth their salt, Fox can smell bullshit from miles away.
So….In Batman Begins we’re introduced to God Morgan Freeman as Lucius Fox, Fox is the research head (and apparently the only researcher) of the Applied Sciences Division arm of Wayne Enterprises. This basically involves watching over all the important and ultra cool aborted research projects and prototypes that Wayne Enterprises came up with over the years but never put into general use (they do this A LOT seemingly).

Fox becomes Batman’s armourer and throughout the trilogy he oversees the construction of two different Batsuit’s. The first in Batman Begins is more cumbersome than that used in the majority of The Dark Knight and The Dark Knight Rises. This second suit actually made Batman’s signature cowl more like a motorcycle helmet (on the right above). This is eminently more practical as Bruce Wayne points out to Fox in The Dark Knight:
Bruce Wayne: I need a new suit.
Lucius Fox: Yeah, three buttons is a little ’90’s, Mr. Wayne.
Bruce Wayne: I’m not talking fashion, Mr. Fox, so much as function.
Lucius Fox: You want to be able to turn your head.
Bruce Wayne: Sure would make backing out of the driveway easier.
The latter bodysuit is made of “hardened Kevlar® plates over titanium-dipped tri-weave fibres for flexibility” and is broken into multiple pieces over the bodysuit for greater mobility than the original neoprene “Survival suit” from Begins that was coated with Nomex® and had Kevlar® armour plates. Now there’s some materials chemistry at work here, so let’s have a little deeper look.

These are Kevlar fibers, NOT yellow candy floss.
Like a lot of modern materials, Kevlar® is a polymer. That’s right folks basically it’s a plastic like most of the military survival “smart suit” that becomes the evening wear of a certain caped crusader.

You can even follow Kevlar on twitter. How modern!
Of course, Kevlar is the trade name of the material (you could probably tell by the ®, right?). Poly-paraphenylene terephthalamide just doesn’t sound quite so catchy especially when you are in the business of actually selling stuff. Kevlar was first isolated in a workable form by Stephanie Kwolek, a polish chemist, whilst working at DuPont in 1964. It was one of those serendipitous discoveries, which only came to light when they investigated a solution that would normally have been thrown away. Instead, finding that it was “cloudy, opalescent upon stirring and of low viscosity”, they convinced those running the “spinneret” to do them a favour and discovered that the fiber that was drawn out did not break, unlike nylon (a related polyamide).

Reels and reels of Kevlar
This generated a whole series of new polymers known as Aramids (a portmanteau of “aromatic polyamide”) – a class of heat-resistant and high strength synthetic fibers. Kevlar was introduced as a product in 1971 and has found widespread use, although most famously as a material in bullet proof vests. Other uses include – as a cryogenic material for superconducting magnets, sports equipment, tires, basketball shoes, audio speakers, tennis racket strings, frying pans (as a substitute for Teflon®), ropes, cables, smart-phone cases, brake pads…I could go on, suffice to say it is a versatile material and there have been 8 different grades used for different purposes.
Of course despite its versatility the use of Kevlar that people generally remember is still in “bullet proof” (or ballistic) vests because of the wonderful P.R. job that Hollywood has done. For instance, it saved Doc Brown (eblow) in Back to the Future, which is a good or bad thing depending on how you felt about those two sequels (I love them for the record!)

Great Scott! Kevlar saved my life! Does this mean I’m really Batman?
The first all Kevlar vest was introduced in 1975, by American Body Armor. It was called the K-15, and unsurprisingly consisted of 15 layers of Kevlar and steel shock plates over the heart. The material continues to be used today, despite many other bullet resistant materials having been developed, in part because by comparison it’s quite cheap. Kevlar stays strong down to even cryogenic temperatures (e.g. even in liquid nitrogen at −196 °C), whilst it starts to lose its strength slowly above about 160°C over a prolonged period of time.
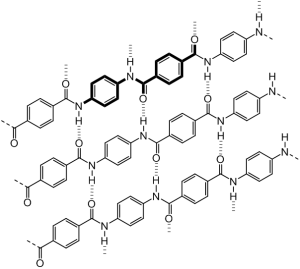
This is part of a polymer chain of “Kevlar”, obviously it takes a LOT more of these for form a massive strand. In Bold is a monomer unit. Imagination time!
The reason Kevlar is such a useful material to Batman is due to this remarkable strength, protecting from knives, bullets and physcial smackdowns. The fibers are about five times stronger than steel on a pound-for-pound comparison. The chains of the polymer are ordered in parallel lines where the benzene rings stack to give a symmetric and highly ordered structure (much like silk) with man inter-chain bonds due to the interaction of the carbonyl and NH groups along the chain (see figure above). Further to this there are aromatic stacking interactions between adjacent strands. These interactions are exceptionally important in giving the material its high tensile strength.
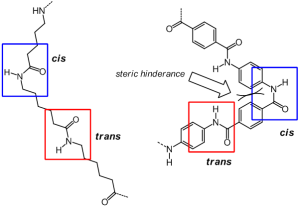
Nylon type chain on the left has little to hinder a cis type amide formation, whereas the hinderance of the bulky aromatic rings makes cis unlikely in Kevlar.
In nylon, the chains are generally more able to twist from a trans (opposite side in Latin) to cis (same side in Latin) peptide geometry. This has the result of royally messing up the nice fiber that we want. Kevlar is different. The cis– conformation is just far too sterically (or spatially) hindered i.e. the hydrogens on the rigid aromatic groups interfere with each other. As such Kevlar remains almost always in the trans– conformation, hence the beautiful fibers.
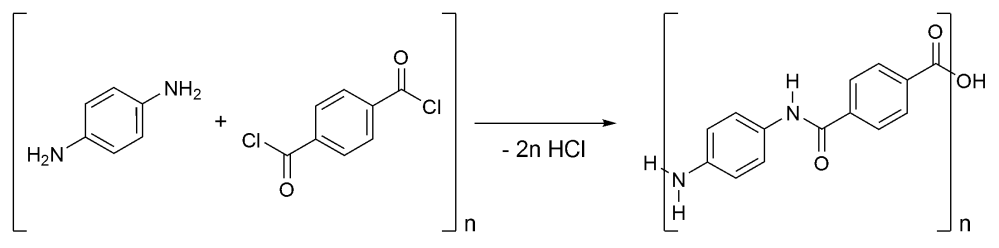
I’ve borrowed this one from wiki as chem draw was being less than helpful in finishing this blog post…
Kevlar is synthesised in solution from the monomers 1,4-phenylene-diamine (1,4- diaminobenzene) and terephthaloyl chloride (1,4-benzenedicarbonyl chloride) in a condensation reaction. The reaction yields two equivalents of hydrochloric acid as a by product. 1,4-phenylene-diamine is a precursor to many aramid plastics and fibers, which exploit the monomers difunctionality allowing the molecules to be strung together. Kevlar is of the simplest AABB type (i.e. it goes one monomer then the other).
The solvent used in the reaction was originally the highly co-ordinating hexamethylphosphoramide (HMPA), which isn’t as toxic as many make out, but it does have a pesky habit of being rather carcinogenic….
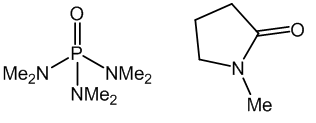
One of these two solvents is not very nice…hint: it’s the one on the left
Generally now, the polymerisation is conducted in a solution of N-methyl-pyrrolidone and calcium chloride. It should be noted that salts and other impurities, such as calcium with can interrupt the hydrogen bonding interactions of the chains and care has to be taken in production to make sure the polymer is as free from these as possible
Once the reaction is complete the brand spanking new polymer is filtered, washed and dissolved in concentrated sulfuric acid (I miss sulphur) and is finally extruded through spinnerets. The string of polymer (or filament) is passed through a narrow passage and goes through the wet spin process where it is coagulated in sulfuric acid. The filament can take different paths; it can be formed into a fine yarn, washed and dried which is wound into spools (see picture!) or it can form filament pulp, spun-laced sheets, paper and obviously Batsuits.
The cowl (in both instances) used by Batman in all of Nolan’s movies, is also made of a Kevlar polymer, reinforced with graphite or carbon-fiber. You could write a whole blog just on this, so I won’t go into detail here. Suffice to say that carbon-fiber-reinforced polymer or carbon-fiber-reinforced plastic is an extremely strong and light fiber-reinforced polymer, although it’s apparently no match for an old man with a mallet…

He’s not SIR Michael Caine for nothing you know.
And it’s not much good against this guy either…

I am Bane. Hear me roar….And stare weirdly and shit.
Moving on to another material mentioned by Fox, in the build of the Batsuit Nomex® is a fire-resistant polymer also devised and marketed by DuPont and is closely related to Kevlar by being an aramid type polymer. In the case of Nomex it is a meta-aramid, derived from the condensation reaction of monomers, m-phenylenediamine and isophthaloyl chloride.
This is Nomex. You will notice that it simply doesn’t stack as nicely or as straightly as Kevlar. This makes it considerably weaker.
Nomex simplycannot align itself as well as the straighter chain of Kevlar, so the filament formed and has poorer strength properties. However, it does have excellent thermal, chemical, and radiation resistance. So you can thank Nomex for helping Batman survive when the Scarecrow lights him on fire in Batman Begins: click here for clip.
It may also have aided Val Kilmer’s Batman from being turned to dust in Batman Forever though, so…swings and roundabouts really.

Sir Not-Apearing-In-This-Blog-Post
The properties of Nomex also make it the perfect protective material for race-car drivers – Batman is also one of these at times, having no respect for the rules of the road naturally.

Racing is quite dangerous. Nomex has helped save many lives. As many as Kevlar has certainly.
Nomex will burn when you hold a flame to it, but it near immediate stops as soon as the heat source is removed, it is inherently flame retardant. Its thick woven structure is (like Kevlar) a very poor heat conductor, allowing you to…say jump out of a window and roll in the rain like a boss.

There be Neoprene in them there pectorals.
The other polymer mentioned is the much simpler, Neoprene (another DuPont material unsurprisingly – this is turning into a DuPont advert). It is a much older, but extremely versatile synthetic rubber that was originally developed as a substitute for natural rubber with better oil-resistant properties. Its other properties include:
- Resistance against degradation from sun, ozone and weather
- Retains strength over over a wide temperature range
- Physically tough
- Fire Resistant burning inherently
- Outstanding resistance to damage resulting from flexing and twisting
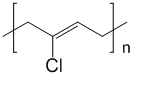
This is the polymer known as neoprene. Where n is a huge number.
The chemical name for neoprene is polychloroprene as shown below. The reaction is initiated via free-radicals, with the monomer being 2-chlorobutadiene. It generally carried out in an aqueous emulsion and has been conducting using a wide range of emulsifying agents such as alkyl sulfonates.

Titanium alloy rods. Make your own joke about rods.
Titanium is apparently also used from The Dark Knight onwards as a coating for the polymer fibers. You’ll find this metal on that chemistry thing known as the periodic table,filed under the symbol Ti and possessing an atomic number of 22. It has a relatively low density, is silver in appearance and is a strong, lustrous and corrosion-resistant metal of the transition series.
It was discovered in by William Gregor from Cornwall, UK in 1791. It is so named for the Titans of Ancient Greek mythology a comment on its strength. It’s pretty common in a range of minerals, and titanium dioxide is the metals most commonly used compound being used in the manufacture of white pigments (you even eat the stuff all the time, creepy yes?). Another compound worth mentioning is titanium tetrachloride (TiCl4) which is a component of smoke screens, but more on that in part 2.

How much do you want to bet there is some titanium in there somewhere?
Titanium’s versatility as a material and its use to the Dark Knight is all due to its ability to form alloys with a large number of other elements. Strong lightweight alloys of titanium have found uses in aeronautics (jet engines, missiles, spacecraft and flying Bat vehicles), industrial processes (chemicals and petro-chemicals, desalination plants, pulp, and paper), dental instruments and fillings, sports equipment, mobile phone parts, and many other applications.
They even made Lt. Dan’s “magic legs” out of titanium.

I’m smiling due to the power of material chemistry….honest.
So by dipping the tri-weave fibers of the suit in titanium, in a high-tech and admittedly unknown process the Batsuit is given some limited amount of the most useful properties of titanium – in particular it has the highest strength-to-weight ratio of any metal on the periodic table, which is pretty useful when fighting crime one imagines.
Add this to the already sterling properties of Kevlar, Nomex and Neoprene and you have something that makes a wet suit look like the equivalent of swimming with Jaws naked.

Yes. It’s “Hollywood Science” time! *jazz hands*
The last thing to mention is that whole “memory cloth” stuff that Batman uses for his cape in the Nolan universe…I’m pretty sure that is Hollywood hokum I’m afraid. There have been some attempts at making self-healing memory materials, but none of this is quite as spectacularly useful as what Lucius Fox invented.

Yeah. I totally believe this.

Truth.
That’s all for now folks. Come back for Part 2 in a couple of weeks to learn about the chemistry behind some of Batman’s gadgets.
Update: Part 2 can be found here.
-Doctor Galactic-




























